SkinPen FAQ
 Vista Dermatology is proud to offer SkinPen® to our clients. As the very first FDA-cleared microneedling device, SkinPen is trusted by leading skincare professionals. Three steps in as little as thirty minutes targets facial acne scars and neck wrinkles, for smoother, more radiant, younger-looking skin.
Vista Dermatology is proud to offer SkinPen® to our clients. As the very first FDA-cleared microneedling device, SkinPen is trusted by leading skincare professionals. Three steps in as little as thirty minutes targets facial acne scars and neck wrinkles, for smoother, more radiant, younger-looking skin.
Here are some frequently asked questions about the procedure.
How does SkinPen work?
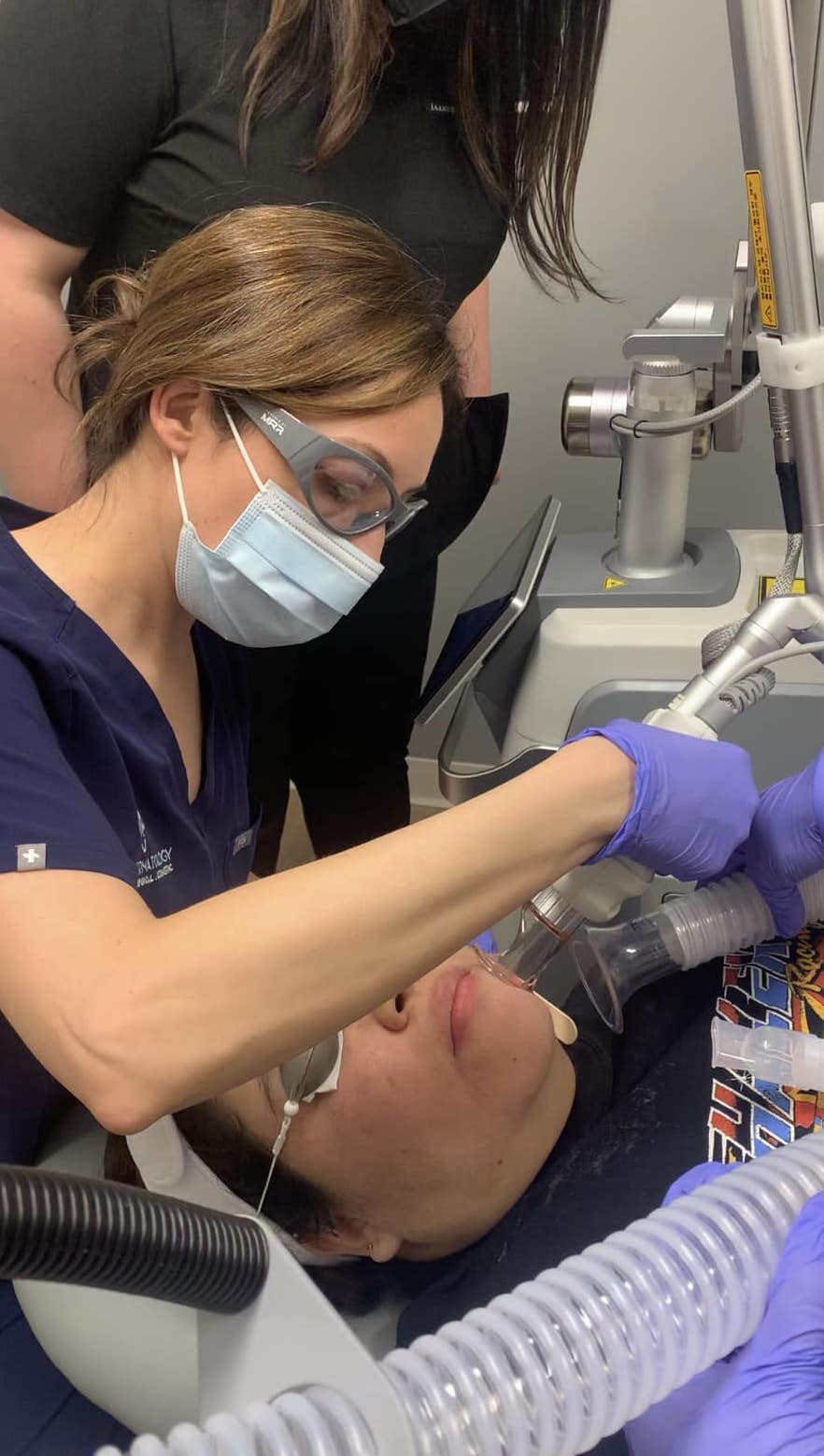
SkinPen creates hundreds to thousands of “micro” skin punctures per second to stimulate the skin’s natural wound healing process – inflammation, proliferation, and remodeling – to prompt tissue remodeling without causing scar tissue formation. Most patients can return to normal activities within 24 hours.
Why should I use it?
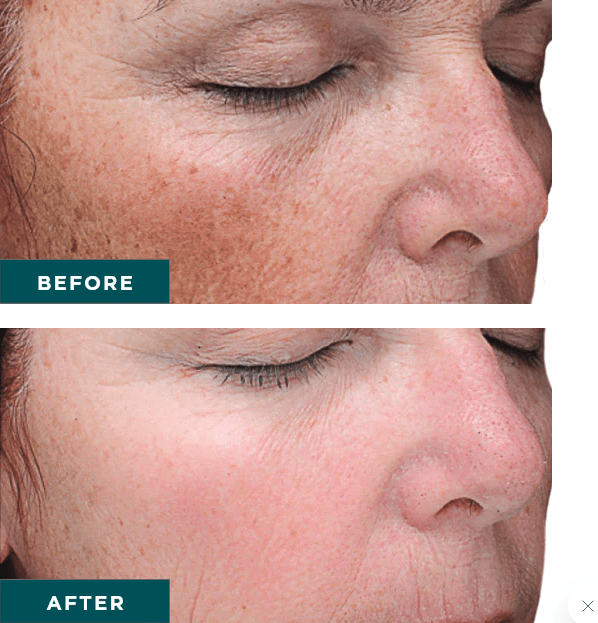
SkinPen is clinically proven to fight the appearance of neck wrinkles and reduce the appearance of acne scars. In fact, 90% of subjects in the clinical trial would recommend the treatment to friends and family. It’s a minimally invasive procedure performed in-office with little to no downtime. As the first FDA-cleared microneedling device, SkinPen sets the industry standard for safety.
Will it work for me?
Unlike some alternatives, SkinPen has been clinically proven to be effective for women and men with with all Fitzpatrick Skin Types I – VI. Likewise, unlike lasers or chemical peels that can damage skin over the long term, SkinPen treatments, when properly spaced and overseen by a physician, can be used for years.
Is it safe?
Yes, by design. SkinPen’s patented – and single-use – sterile needle cartridge is built with safety in mind. SkinPen is also surrounded by a custom-designed BioSheath that acts as a barrier to prevent cross contamination between procedures. That’s part of the reason SkinPen by Crown Aesthetics is the world’s first FDA-cleared microneedling device.
Who shouldn’t use it?
SkinPen should not be used on patients who have active skin cancer in the treatment area(s); open wounds, sores, or irritated skin in the treatment area(s); an allergy to stainless steel or anesthetics; a hemorrhagic (bleeding) disorder or hemostatic (bleeding) dysfunction; are pregnant or nursing; or are currently taking drugs with the ingredient isotretinoin (such as Accutane). Talk to us to see if this treatment is right for you.
What about the procedure did the FDA clear?
Quite a bit. The U.S. Food and Drug Administration’s clearance covers not only the SkinPen device but the entire protocol. That includes the Skinfuse® Lift HG hydrogel used to protect against abrasion and friction during the treatment, as well as the custom-designed BioSheath to prevent cross contamination.
Find out if SkinPen is right for you. Contact us for a consultation.